
Table of Contents
🧬 The Pharma Industry Is Evolving Fast. Are You Ready for Pharma 5.0?
From R&D pipelines to personalized medicine, the pharmaceutical sector is at a tipping point. As clinical trials grow costlier and regulatory complexities mount, pharma companies are turning to Generative AI and Agentic Intelligence not as experiments—but as essential operating levers.
Ajit Mishra, a former Microsoft executive and one of the world’s foremost voices in enterprise AI transformation, calls this evolution Pharma 5.0—a decisive leap from digital enablement to autonomous, AI-driven reengineering.
His latest article, “Pharma 5.0 | 20 Ideas of Digital Transformation in the Pharma Sector with Generative AI,” outlines a strategic roadmap for industry leaders who want to rethink the way pharma works—from molecules to market.
🎧 Prefer audio? Listen to the latest episode of the CAIO Zone Podcast, where this vision comes alive in a 40-minute immersive breakdown.
🧪 Pharma 5.0: Key Ideas That Are Already Shaping the Future
Ajit’s article covers 20+ use cases, but here are a few that every CAIO, CIO, and CXO should understand deeply:
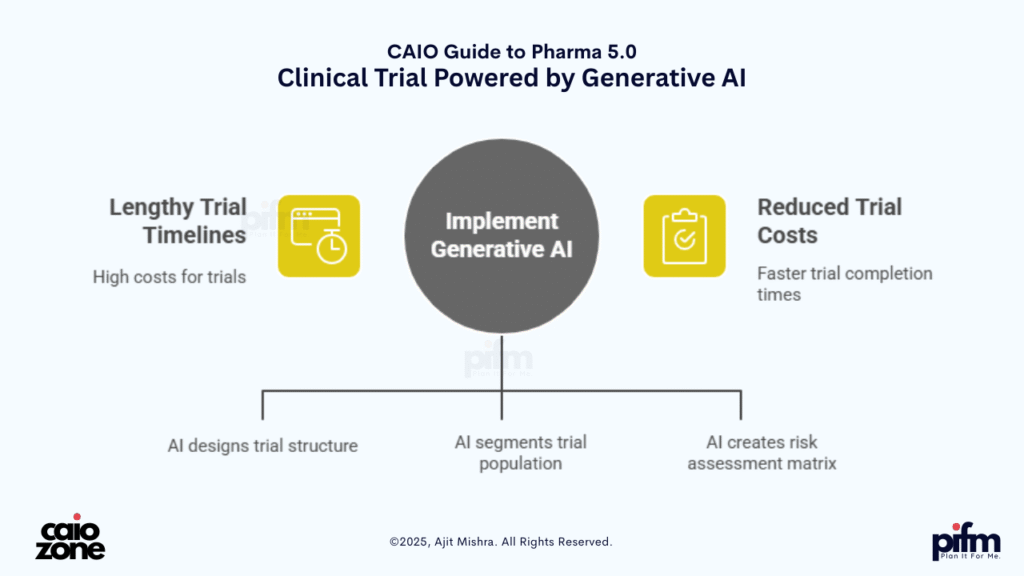
🔍 1. Autonomous Clinical Trial Design
Imagine a system that generates your Phase-II trial structure, population segmentation, and risk matrix on the fly, optimizing protocols across geographies. That’s no longer fantasy. It’s Generative AI in action—dramatically reducing both trial timelines and costs.
Clinical trial design has traditionally been slow, siloed, and manual—often taking months to finalize protocols, recruit participants, and comply with cross-border regulatory requirements. With the advent of Pharma 5.0, Generative AI is reshaping this landscape by enabling on-demand creation of trial designs based on multimodal data and past trial intelligence.
Using enterprise-trained LLMs and structured clinical datasets, AI can now generate:
- 📋 Phase II/III trial blueprints
- 👥 Population segmentation matrices
- 🌍 Region-specific regulatory overlays
- ⚖️ Risk-benefit frameworks and adaptive arms
- 📈 Timeline and resource optimization plans
These designs are not static documents—they evolve. Agentic AI monitors real-time trial data (e.g., dropouts, SAE reports, regional performance), and proposes amendments mid-trial. The outcome is a living protocol—dynamic, contextual, and compliant—leading to faster execution, reduced costs, and smarter drug development decisions.
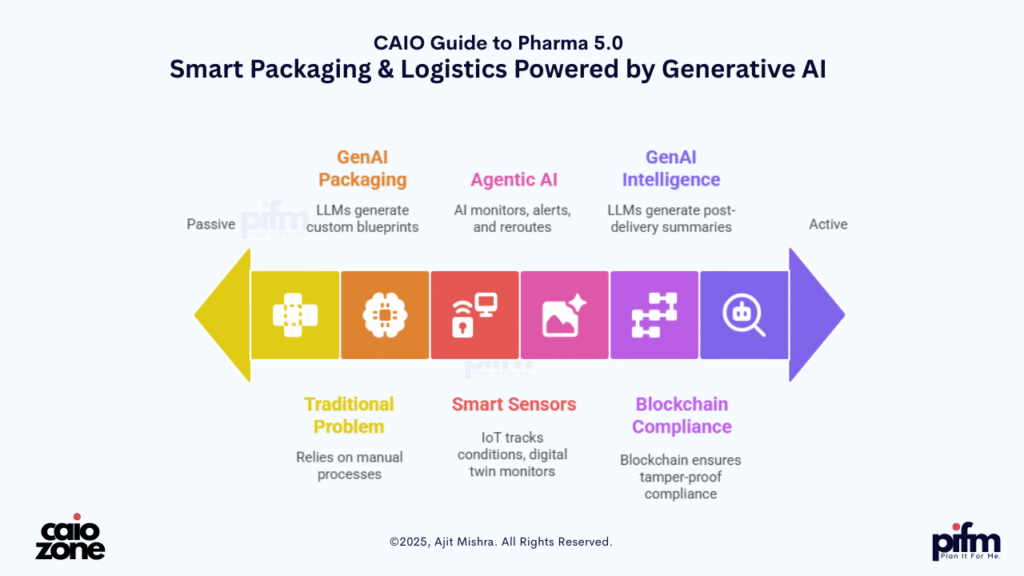
📦 2. AI-Powered Smart Packaging & Logistics
In the Pharma 5.0 era, packaging and logistics are no longer passive endpoints—they are active intelligence layers that ensure authenticity, compliance, and efficiency from lab to last mile.
🧠 Traditional Problem:
- Cold-chain drugs (like mRNA vaccines) are sensitive to temperature and humidity.
- Global pharma shipments face theft, counterfeiting, and tampering.
- Compliance requires full-chain documentation, serialization, and audit trails.
🔁GenAI-Generated Packaging Protocols
- Input: Product type, regulatory zone, environmental sensitivity, shipping partner profile.
- Output: Custom smart packaging blueprint generated using LLMs trained on regulatory + logistics datasets (e.g., GDP, US FDA, EU MDR, WHO PQ).
- Example: For a biologic drug requiring 2–8°C, the GenAI generates packaging material specifications, SOPs, and labeling designs—including language localization.
📦Embedded Smart Sensors & Digital Twins
- Each package includes IoT sensors that track temperature, humidity, light exposure, and tamper detection.
- A Digital Twin of the shipment is created in real time, monitored by Agentic AI systems.
🔍Autonomous Logistics Monitoring by Agents
- Agentic AI tracks route changes, customs delays, and potential cold-chain breaches.
- If a package exceeds the safe temperature range, the agent can:
- Trigger auto-alerts to logistics teams and healthcare providers
- Generate regulatory deviation reports instantly
- Recalculate alternate delivery routes or storage hubs
🛡️Blockchain-Linked Provenance & Compliance
- Every packaging event—manufacture, seal, scan, shipment—is recorded via blockchain for tamper-proof compliance.
- Agents generate audit-ready reports with e-signatures, supporting global GxP documentation needs.
🔗 GenAI for Post-Market Intelligence
- Post-delivery, packaging data is fed into LLMs to generate summaries:
- Trends in logistic delays
- Temperature violation clusters
- Risk predictions for certain lanes or vendors
🎯 Key Value Proposition:
- ✅ Ability to scale just-in-time distribution for critical drug programs
- ✅ Real-time compliance assurance with zero manual logging
- ✅ Reduced product recalls and temperature excursion losses
- ✅ Increased trust among regulatory bodies and healthcare providers
- ✅ Enhanced pharmacovigilance through continuous packaging analytics
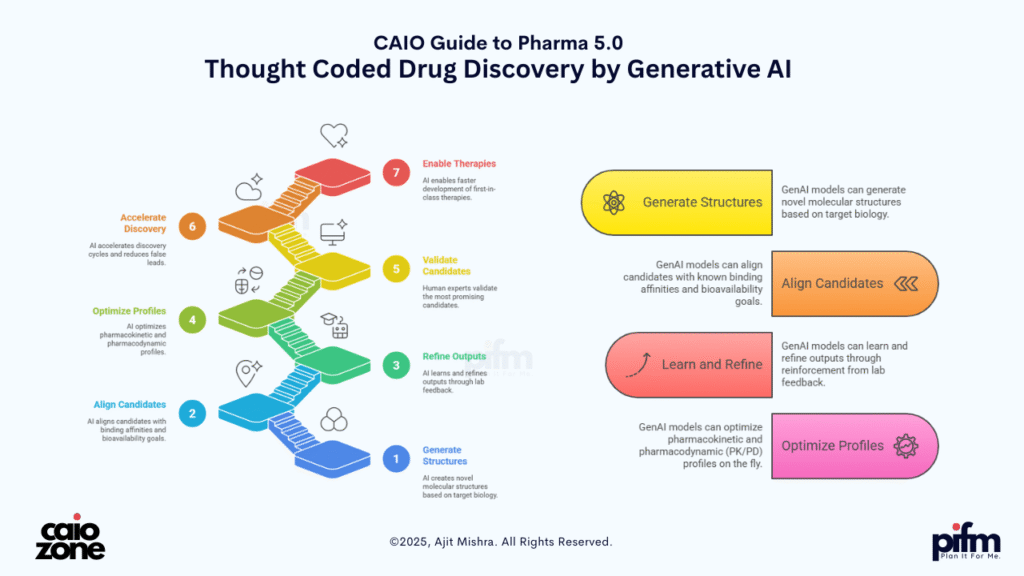
🧠 3. Thought-Coded Drug Discovery
Drug discovery has historically relied on years of trial-and-error in wet labs, with chemists manually designing molecules, running simulations, and waiting on assay feedback. But in the Pharma 5.0 paradigm, Ajit Mishra introduces a bold shift—from molecule discovery to molecule co-creation using Generative AI and cognitive reasoning.
Instead of searching for potential compounds, GenAI models can now:
- 🧪 Generate novel molecular structures based on target biology
- 🎯 Align candidates with known binding affinities and bioavailability goals
- 🔁 Learn and refine outputs through reinforcement from lab feedback
- 🧬 Optimize pharmacokinetic and pharmacodynamic (PK/PD) profiles on the fly
The result? A cognitive lab that thinks with the scientist—not just for them. These AI-powered systems act like digital chemists—proposing, modifying, and validating compound structures through simulation and predictive analytics. Human experts then validate only the most promising candidates, dramatically accelerating discovery cycles, reducing false leads, and enabling first-in-class therapies faster than ever.
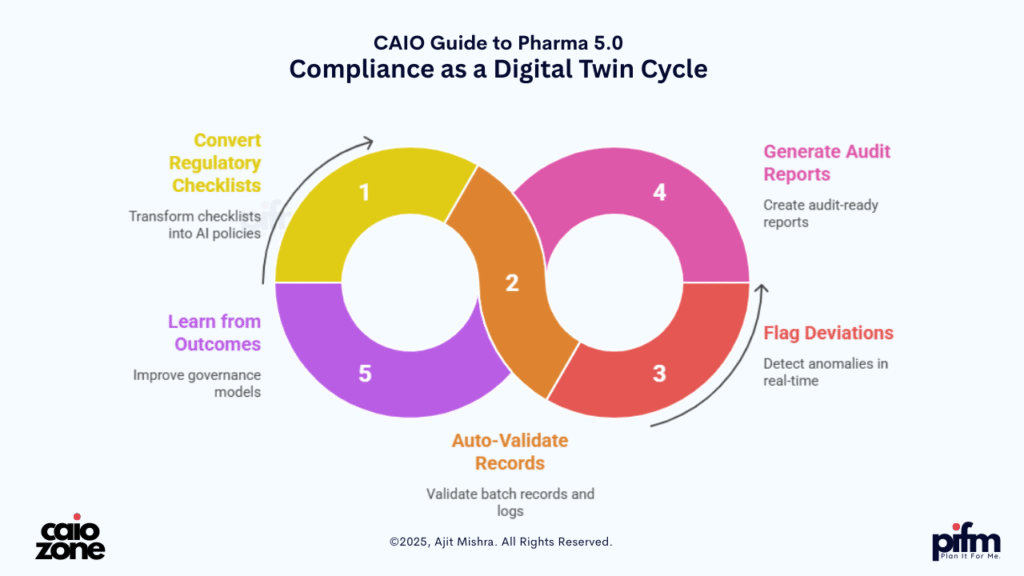
🛡️ 4. Compliance as a Digital Twin
Compliance has always been the most documentation-heavy, risk-averse function in pharma. Whether it’s FDA CFR 21 Part 11, ICH GxP, or EMA protocols, ensuring regulatory alignment across geographies requires constant monitoring, manual validation, and retrospective audits. But Pharma 5.0 introduces a transformative idea—Compliance as a Digital Twin—where AI codifies regulatory logic into real-time autonomous agents.
With this shift, organizations can:
- 📜 Convert regulatory checklists into machine-readable AI policies
- 🚦 Auto-validate batch records, audit trails, and system logs
- 📍 Flag real-time deviations or anomalies during production
- 🔄 Generate audit-ready reports with e-signature workflows
- 🧩 Continuously learn from inspection outcomes to improve governance models
These Compliance Twins act like invisible auditors—operating 24/7 across manufacturing, lab operations, and distribution layers. They not only detect non-conformance early, but also suggest corrective actions, generate deviation justifications, and simulate impact assessments—before humans even intervene. The result is a move from risk mitigation to risk anticipation, making compliance faster, cheaper, and future-proof.
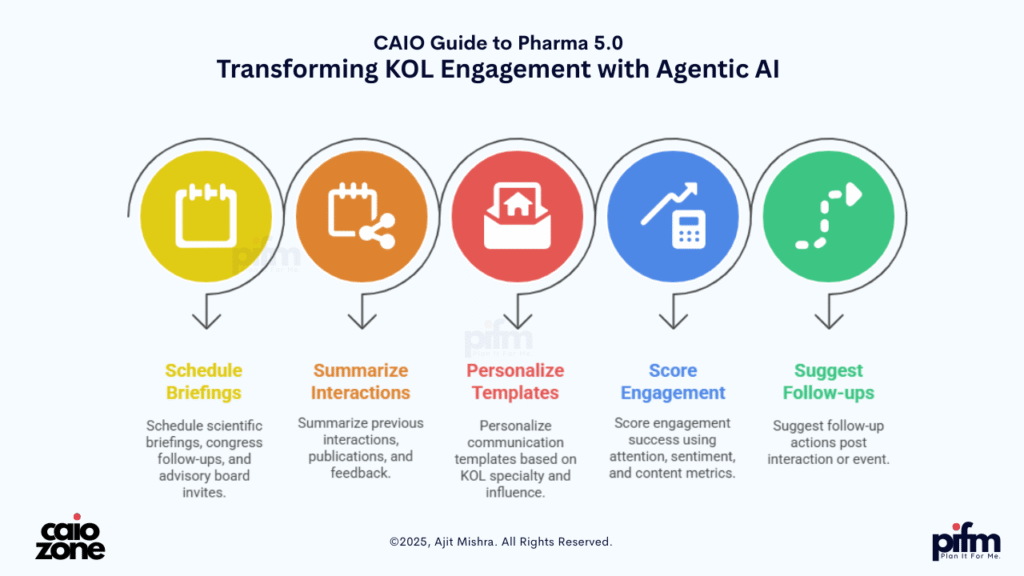
👥 5. Agentic AI for KOL Engagement
(Intelligent orchestration of scientific relationships)
Engaging with Key Opinion Leaders (KOLs) is central to pharma’s scientific credibility and market influence. Yet, managing these relationships through traditional CRMs is manual, inconsistent, and often lacks personalization. In Pharma 5.0, Agentic AI transforms KOL engagement into an intelligent, automated, and insight-driven process—managed by autonomous copilots that work in the background.
These agentic copilots can:
- 📆 Schedule scientific briefings, congress follow-ups, and advisory board invites
- 📝 Summarize previous interactions, publications, and feedback
- ✉️ Personalize communication templates based on KOL specialty and influence
- 📊 Score engagement success using attention, sentiment, and content metrics
- 🔁 Suggest follow-up actions post interaction or event
Instead of relying on fragmented CRM notes and sales reps, organizations can deploy always-on digital relationship managers who engage KOLs at scale with surgical precision. These agents can even auto-detect scientific trends from KOL publications and trigger insights for Medical Affairs and Commercial teams. The outcome is stronger scientific relationships, faster feedback loops, and deeper influence—without increasing human overhead.
🎙️ The Podcast Breakdown: Listen to Pharma 5.0 Come Alive
Ajit’s latest episode on the CAIO Zone Podcast distills the heart of Pharma 5.0 into an engaging audio format. Whether you’re on the move or want a teaser before reading the full article, this episode is your perfect primer.
▶️ Title: Pharma 5.0 | Reengineering the Pharma Sector with Generative AI
⏱️ Duration: ~40 mins
🎙️ Host: CAIO Zone Podcast
🔗 Listen on YouTube | Listen on Spotify
📖 Read the Full Article on Medium
If these ideas sparked your curiosity, the full article delivers 20 actionable insights with real-world relevance—from R&D and manufacturing to supply chain and compliance.
🧭 Ready to explore all 20 ideas?
👉 Read Ajit Mishra’s full Pharma 5.0 article on Medium »
🔮 Final Thoughts
Pharma 5.0 is not about moonshots—it’s about precision transformation, engineered by Chief AI Officers who understand both science and systems.
Whether you’re a pharmaceutical executive, technology leader, or AI professional, Ajit Mishra’s frameworks and playbooks are redefining the industry. Now is your time to act.
✨ Subscribe to the CAIO Zone Newsletter to stay ahead in Pharma, Healthcare, and Life Sciences innovation.











